Line clearance is a critical process in pharmaceutical manufacturing, ensuring that no materials from a previous batch contaminate the next. However, this process is traditionally time-consuming, prone to errors, and a source of inefficiency.
Five years ago, Catalyx set out to improve production and packaging line operations for life sciences companies through digital transformation, despite it being a complex proposition in the slow-to-change pharmaceutical world. Said Elliot Abreu, senior vice president of Catalyx, at PACK EXPO East, “During a customer forum, an executive from a top 10 pharmaceutical company commented that line clearances were possibly the biggest headache facing them personally and their business globally. The impact of quality investigations, deviations, CAPAs, and worst of all, the risk associated with recalls of product led this individual to comment to our CEO that this was easily a $300 million a year problem for them.”
Effects on OEE
Catalyx went on to complete a line changeover survey of life science professionals (reportedly the world’s first) in mid-to-late 2022. The feedback from the customer base included:
- 73% of respondents had experienced at least one line clearance error in the previous 12 months.
- 71% said that undetected “rogue components” that were missed during line clearance were the cause of the line clearance failures.
- 80% of respondents said that the line clearance process was taking in excess of 60 minutes, with 35% reporting that a same product, same size, different language changeover was taking between 90 minutes to three hours to complete.
Abreu said these problems are widespread because existing filling and packaging lines were built to optimize the speed of delivery of the mega batch. Complex machines can deliver accuracy and large batches of product but can often take many hours to changeover to the next product batch or order due to size and complexity. “As the market shifts to a greater number of smaller batches, the result is a significant increase in the number of line changeovers being run per day. …Customers report that changeovers are now frequently taking longer to complete than the batch, meaning that lines are spending more time changing over than producing actual product.”
Companies may get stuck in a “line clearance vicious cycle” in which deviations drive corrective actions, which ultimately add complexity to the process, reduce efficiency, and place greater time pressures to meet targets. Depending on complexity, the cost of a deviation can range from the low thousands to hundreds of thousands of dollars.
Operator health and safety must also be considered, and line clearance can involve contorting, stretching, reaching to view areas which may be hard to view or offer low light/visibility.
It goes without saying that beyond the OEE impacts, patient safety is paramount. Depending on manual inspection by operators leaves room for subjectivity and potential errors.
Digitalizing the process
Through the introduction of its Digital Line Clearance Assistant, Catalyx has demonstrated the potential to reduce inspection times by 85% and line changeover times by up to 20%, addressing inefficiencies inherent in manual line clearance processes, giving back valuable production time to pharmaceutical companies. Increased uptime means more batches produced on each line per year.
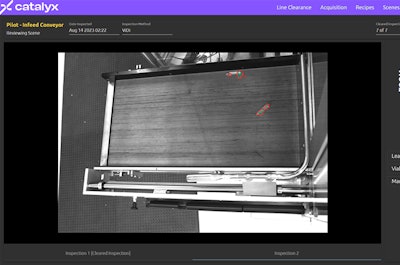
The technology is also designed to improve the quality and consistency of line clearance processes. By using a network of IoT cameras, the system can immediately and simultaneously evaluate whether each area of the production line is clear of rogue components. This objective, repeatable process reduces the risk of human error and ensures a higher standard of quality control.
Further, the system offers digital traceability of all inspections and operator activity, and positively impacts compliance and environmental health and safety. The technology's ability to automatically capture and store evidence of line clearance activities supports regulatory compliance and reduces the risk of costly product recalls.
By minimizing the need for manual inspections, the system protects employees from repetitive actions that can cause workplace injuries. “In an area where employee retention can be a challenge and where experienced line clearance technicians are at a premium, any opportunity to improve the experience of employees can make a difference,” said Abreu.
While the tech can be retrofitted to existing lines, Catalyx is also working with OEMs to bring lines to sites with LCA already installed and ready to use.