Medical device and pharmaceutical packaging is a necessity to keep products sterile and ready for consumer use. But what happens when there is a risk that the packaging itself can harm the device or drug?
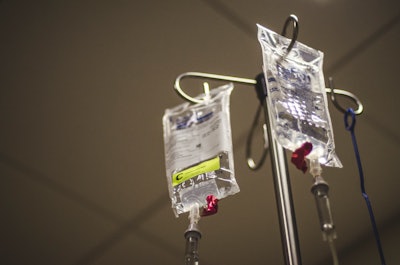
New research cited in a recent article from Packaging Insights states that that is the case for IV bags, claiming that microplastics are present in (intravenous) IV infusions used in medical treatments.
The research, published in the ACS partner journal Environment & Health, found that, after filtering, infusion solutions from PP bottles contain thousands of plastic particles.
“The scientists estimate that a single 250 mL IV infusion bottle could introduce thousands of microplastics — ranging from 1 to 62 micrometers in length — directly into a patient’s bloodstream.”
What I found surprising is that the article states that the smaller the particles, the bigger the danger.
“It’s important to note that smaller particles potentially pose greater hazards. While most particles we measured were between 1–10 μm, even smaller nanoplastics have greater penetration capabilities and may even cross the blood-brain barrier, reaching more critical organs in the human body,” says Liwu Zhang, one of the leading researchers in the study.
The research is meant to shine a light on the medical packaging industry and encourage developers to explore alternative materials or processes that could eliminate this threat while still offering the devices the protection they need.